A new type of HIV vaccine that trains the immune system to produce broadly neutralizing antibodies (bnAbs) looks promising in an early study, researchers recently reported in Science. However, it will be years before this approach could be tested in large clinical trials and deployed worldwide.
Almost all participants in the small Phase I IAVI G001 trial who received an initial priming vaccine produced bnAb precursors, which worked better after a booster shot. The results establish proof of concept that this approach, known as germline targeting, warrants further development, the researchers concluded.
These data “demonstrate for the first time that one can design a vaccine that elicits made-to-order antibodies in humans,” lead study author William Schief, PhD, director of IAVI’s Neutralizing Antibody Center at Scripps Research, said in a press release. “We believe this vaccine design strategy will be essential to make an HIV vaccine and may help the field create vaccines for other difficult pathogens.”
Researchers have spent more than three decades and billions of dollars studying vaccines to prevent and treat HIV, with little success. The virus mutates rapidly, and there are many different strains around the world, making it difficult to develop broadly effective vaccines.
To date, only one HIV vaccine regimen—a canarypox vector primer followed by a gp120 envelope protein booster—has demonstrated partial protection in a human study, but it was not effective in the larger Uhambo trial. Two ongoing trials, Mosaico and Imbokodo, are testing an adenovirus primer followed by a booster that contains a mosaic of proteins from multiple HIV strains.
Scientists at IAVI, the National Institute of Allergy and Infectious Diseases Vaccine Research Center, Scripps Research and the Fred Hutchinson Cancer Center are taking a different approach.
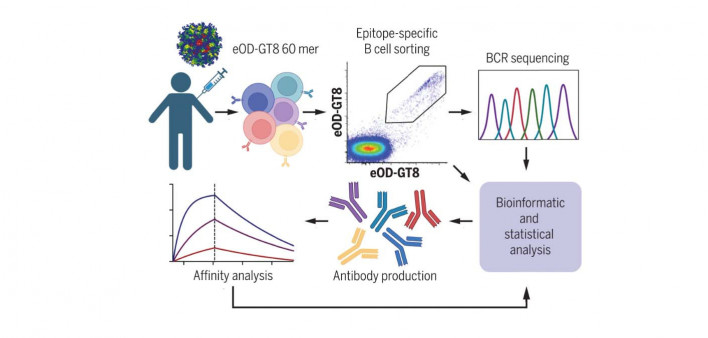
Germline targeting aims to train the immune system to generate B cells that can make broadly neutralizing antibodies. People living with HIV normally produce antibodies against the virus, but they usually target parts that are highly variable, so they don’t recognize new viral mutations. However, a small number of people naturally produce bnAbs that target hidden conserved parts of the virus, and most people possess rare immature B cells that are potentially capable of doing so. Germline targeting uses a series of vaccines in a stepwise manner to spur the development of these specialized B cells and train them to produce bNAbs.
The Phase I IAVI 001 trial (NCT03547245)—the first human study of this approach—tested eOD-GT8 60mer, a so-called immunogen consisting of 60 engineered copies of HIV’s gp120 envelope protein genetically fused to a nanoparticle. The immunogen is designed to encourage specialized B cells to production of bnAbs similar to VRC01, which has been tested in several studies. Preclinical studies showed that eOD-GT8 60mer plus an adjuvant (a substance that enhances immune response) stimulated VRC01-class B-cell responses in mice.
Schief presented initial results from IAVI 001 at the 2021 HIV Research for Prevention Conference (HIVR4P). Although the news received little attention at the time, a flurry of social media in April gave the impression that the research is further along than it is. Detailed findings from the study have now been published in the December 2 issue of Science.
Schief and a large team of collaborators enrolled 48 healthy HIV-negative adults at Fred Hutch in Seattle and George Washington University in Washington, DC. The participants were randomly assigned to receive either two injections of eOD-GT8 60mer plus an immune-boosting adjuvant (AS01B), at a low or high dose) or placebo injections, spaced two months apart.
The vaccine stimulated the production of specialized precursor B cells, the first step in the pathway for generating bnAbs. In fact, 35 of the 36 people (97%) who received eOD-GT8 60mer produced the desired precursor B cells while only two placebo recipients did so. The bnAb purcursors made up a median 0.1% of memory B cells in the blood.
“We specified in advance certain molecular properties of the antibodies that we wanted to elicit, and the results of this trial show that our vaccine antigen consistently induced precisely those types of antibodies,” Schief said.
After the booster shot, these B cell precursors produced antibodies with greater affinity for the HIV envelope proteins. The vaccine had a favorable safety profile with no serious side effects.
The study also examined in detail the properties of the antibodies and B cells induced by the vaccine, according to the Scripps press release. One analysis showed that the immunogen first stimulated an average of 30 to 65 different bnAb precursors per vaccinated participant and then caused those cells to multiply. Other analyses looked at specific mutations the bnAb precursor B cells acquired over time and how tightly they bound to the immunogen, showing that after each dose of the vaccine, “the bnAb-precursor B cells gained affinity and continued along favorable maturation pathways.” While a majority of precursor B cells triggered by the vaccine were not capable of producing bnAbs, but bnAb precursors had greater binding strength.
Moving Forward
While promising, this is just the first stage of what is expected to be a multistep vaccine regimen that elicits bnAbs with increasingly affinity for HIV. So far, the researchers have not tested antibody responses against HIV, much less whether the vaccine prevents HIV acquisition in the real world.
Experts hope the mRNA technology used in the Moderna and Pfizer-BioNTech COVID-19 vaccines can help speed up and lower the cost of HIV vaccine development.
The mRNA vaccine technology uses lipid nanoparticles, or fat bubbles, to deliver bits of genetic material that encode instructions for making proteins. The mRNA COVID-19 vaccines, for example, deliver blueprints for making the SARS-CoV-2 spike protein. Germline targeting is expected to require numerous steps using multiple vaccines. Because the mRNA genetic code in a vaccine can be easily swapped out, researchers will use the technology to rapidly generate and deliver successive versions of the HIV immunogen.
The IAVI G002 trial (NCT05001373), now underway at four sited in the United States, is testing the eOD-GT8 60mer immunogen delivered via mRNA (dubbed mRNA-1644) and a related candidate developed by scientists at Scripps Research, IAVI and Moderna. The company announced in January that participants in the trial had received their first vaccine doses. A companion study, IAVI G003 (NCT05414786), is underway in Rwanda. A third trial, HVTN 302 (NCT05217641), underway at ten U.S. sites, is testing mRNA delivery of three stabilized HIV trimers that could be used in vaccine regimens to stimulate nbAbs.
If these studies pan out, it will be the first step in a years-long process of evaluating the vaccine regimen in larger clinical trials. What’s more, as Penny Moore, PhD, of the National Institute for Communicable Diseases in Johannesburg, noted in a perspective accompanying the Science report, “[T]the incorporation of many different shots into an HIV vaccine regimen is unappealing. Getting the balance right between the need for antibody maturation toward bnAbs and feasibility in the real world will be essential.”
These studies are leading toward a preventive vaccine, but a therapeutic vaccine that generates nbAbs could potentially help bring about long-term remission in people living with HIV. “[I]f we get it to work well enough, we could also give the vaccine to people on ART [antiretroviral therapy], which might eventually allow them to go off ART,” Schief told POZ.
Click here to read the study abstract.
Click here for more news about HIV vaccines.
Click here for a POZ feature on HIV vaccine development.






Comments
Comments